Super-resolution microscopy
Super-resolution microscopy
Super-resolution microscopy is a series of techniques in optical microscopy that allow such images to have resolutions higher than those imposed by the diffraction limit, which is due to the diffraction of light. Super-resolution imaging techniques rely on the near-field (photon-tunneling microscopy as well as those that use the Pendry Superlens and near field scanning optical microscopy) or on the far-field. Among techniques that rely on the latter are those that improve the resolution only modestly (up to about a factor of two) beyond the diffraction-limit, such as confocal microscopy with closed pinhole or aided by computational methods such as deconvolution or detector-based pixel reassignment (e.g. re-scan microscopy, pixel reassignment), the 4Pi microscope, and structured-illumination microscopy technologies such as SIM and SMI. There are two major groups of methods for super-resolution microscopy in the far-field that can improve the resolution by a much larger factor: Deterministic super-resolution: the most commonly used emitters in biological microscopy, fluorophores, show a nonlinear response to excitation, which can be exploited to enhance resolution. Such methods include STED, GSD, RESOLFT and SSIM. Stochastic super-resolution: the chemical complexity of many molecular light sources gives them a complex temporal behavior, which can be used to make several nearby fluorophores emit light at separate times and thereby become resolvable in time. These methods include super-resolution optical fluctuation imaging (SOFI) and all single-molecule localization methods (SMLM), such as SPDM, SPDMphymod, PALM, FPALM, STORM, and dSTORM. On 8 October 2014, the Nobel Prize in Chemistry was awarded to Eric Betzig, W.E. Moerner and Stefan Hell for "the development of super-resolved fluorescence microscopy", which brings "optical microscopy into the nanodimension". The different modalities of super-resolution microscopy are increasingly being adopted by the biomedical research community, and these techniques are becoming indispensable tools to understanding biological function at the molecular level.
Read more about 'Super-resolution microscopy' at: WikipediaWikipedia contributors. "Super-resolution microscopy." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, Feb. 9, 2026.
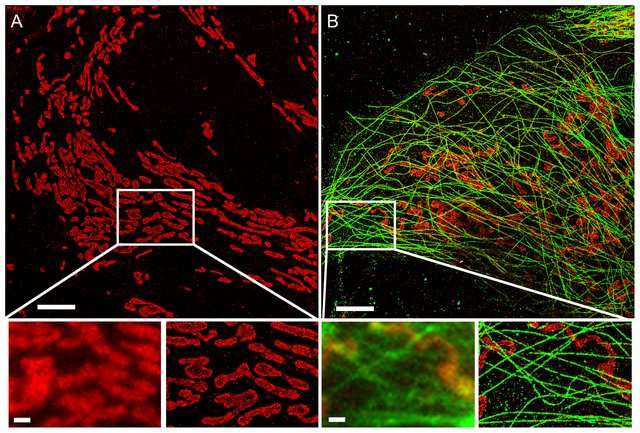
The diffraction barrier is a fundamental physical limit to the resolution that can be achieved with conventional microscopy. There are, however, several techniques that have managed to overcome this limit through applying clever changes to the way images are acquired. Popular representatives (non-exhaustive list!) are Fluorescence Photoactivated Localization Microscopy (PALM), Stochastic Optical Reconstruction Microscopy (STORM), Point Accumulation in Nanoscale Topography (PAINT) and Stimulated Emission Depletion (STED). PALM, STORM and PAINT rely on stochastic processes to reconstruct the position of single fluorophores. If fluorophores are too close together, the point spread function of the imaging device will make their distinction impossible. However, if only one of the neighboring fluorophores is active at a time, its position can be estimated very accurately through fitting a Gaussian intensity model to the acquired images. By stochastically switching individual fluorophores on and off while taking a sequence of images, individual molecules can be localized precisely. PALM, STORM and PAINT use different techniques to generate stochastic activation of an optically resolvable subset of fluorophores. STORM and PALM use photoswitchable fluorophores that can be photoactivated and subsequently excited with an excitation laser. PAINT uses molecules that transiently bind to target structures and get activated in the process.
STED actively deactivates fluorophores in a doughnut shape around the illuminated area using a depletion laser, thus effectively reducing the area in which fluorophores can be excited and emit fluorescent light.
Publications
Single-molecule localization microscopy
Lelek M, Gyparaki M, Beliu G, Schueder F, Griffié J, Manley S, Jungmann R, Sauer M, Lakadamyali M, Zimmer C - Nature Reviews Methods Primers - 2021